"If 2 jabs don't work, it doesn't mean that the jabs might not work, but that more jabs need to be given. "
After Pfizer had received permission in both the United States and Europe to offer its vaccines to children between the ages of 5 and 12 under emergency use authorization, it was time to tap into a new market: The babies and toddlers. To this end, thousands of parents were recruited to let their children, between 6 months and 5 years old, participate in an experiment for a vaccine against a disease that these children themselves experience virtually no problems with. In the end, 4526 children, of which 1776 children under 2 years old, were found who did not yet have antibodies against Corona and whose parents were willing to sign them up for this experiment.
The vaccine works great... according to Pfizer
On May 23, Pfizer sent a press statement containing the results of the trial. These were great. A vaccine efficacy of 80.3%, strong immune response and good safety profile. Quite a feat for a vaccine that has been developed for a completely different variant than the virus that is currently circulating.

Pushback from the HART group
Two weeks ago, however, there was a stir on social media due to a video made by Dr. Clare Craig of the Heart Group from the United Kingdom. She pointed out many irregularities in the Pfizer trial for these children. Naturally, all sorts of self-proclaimed fact-checkers flew on this video to try and debunk her claims. Last week I hit one of the main (500K+ views) in conversation. Regardless of what you may think of these people whose main goal seems to be to ridicule scientists with a message they don't like; Such a conversation is useful to see the other side and thus to look at such a video with a more objective view. After all, if there is something wrong with the message they are agitating against, you can assume that they will bring it forward and magnify it explicitly. I therefore want to use Clare's message as a starting point in the post to see if her claims are actually correct.
Let's take a look at the claims Dr. Craig makes here:
- The trial involved 4,526 children between the ages of six months and four years. 3,000 of these children did not make it to the end of the trial
- They defined severe COVID as children with a slightly elevated heart rate or a few breaths per minute; there were six children, ages two to four, who had severe COVID in the vaccine group, but only one in the placebo group. Based on this, you could argue that the vaccine causes Covid.
- There was one child who was hospitalized, he had a fever and a seizure. It was vaccinated.
- They waited 3 weeks between the 1st and 2nd dose. In those 3 weeks, 34 children who had received the vaccine contracted Corona compared to 13 in the placebo group. That means a 30% higher chance of getting Corona if you are vaccinated. So that data was ignored.
- After that, there were 8 weeks between the 2nd and 3rd dose, during which again many vaccinated children got Corona. That data was also ignored.
- The few weeks after the 3rd dose were also ignored, which meant that in the end they had ignored 97% of all Corona cases and they were looking at a very very small number of cases. 3 in the vaccine group versus 7 in the placebo group
- In the 2 months that followed, they looked at how many children got Corona again. There were 12 of them, 11 of which were in the vaccine group
- If we look at safety: these children were followed for 6 weeks. After that, the blinding was lifted and the children in the placebo group were vaccinated. There goes your security check. Lost forever.
The fact check
In her video, Dr. Craig suggests that the information she gives comes from the trial results of Pfizer, but the link is not given anywhere. However, Google is your friend in this. These are this document of the FDA, which describes Pfizer's trial results. It contains several tables with the results and I will refer to these tables frequently. We'll start at the front.
Claim 1 ‚Äď 4562 participants, 3000 didn't make it to the end
In the study results, Pfizer has made a distinction between babies and toddlers (younger than 2 years) and toddlers and preschoolers (2 to 5 years).
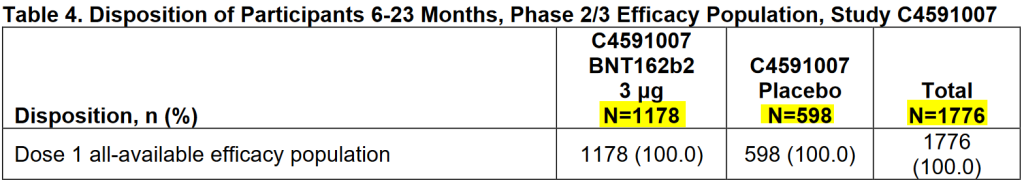



If we add up, we arrive at 4526 children who participated in this trial, of which only 1415 (555 + 860) children still participated in the trial for the 3rd doses. More than 3000 children, more than 2/3rd of the population, were not included in the final results. So far, so good, Dr. Craig's story is true. Another thing that stands out, something Dr. Craig didn't mention, is the ratio of children who have had the vaccine to children who have had the placebo. It's 2:1. 1:1 is common and therefore you automatically assume that if she mentions absolute numbers and they are higher for the vaccine group than for the placebo group, the risk for the vaccine group is greater. That doesn't have to be true. It's not a lie from Dr. Craig. A bit sloppy, though.
Then Dr. Craig makes a very big thing out of the fact that 2/3rds of the population didn't finish the trial: "Why was this? It must be answered! On this basis alone, the trial results are invalid." However, the document simply states why these children did not participate in the final phase. On page 16: "Based on analyses of post-Dose 2 safety and effectiveness data, the protocol was amended to add a third primary series dose at least 8 weeks after Dose 2 (C4591007 protocol amendment 6). Participants enrolled prior to implementation of protocol amendment 6 (N=3,883; February 1, 2022), were able to be unblinded at their 6-month post-Dose 2 visit, and those originally randomized to placebo were offered BNT162b2 vaccination at the age-appropriate dose level."
So the protocol was adjusted during the study. However, it had already been established that in children the blinding would be lifted after x weeks and the placebo group would still receive the vaccine. So the fuss Dr. Craig makes about it is unnecessary and doesn't do her story any good. That doesn't mean that there shouldn't be a fuss about this. The reason for the amendment of the protocol is something that should set alarm bells ringing. What it actually says here is that Pfizer had thought that 2 injections would be enough to get the desired data for effectiveness. And that was disappointing. And not a little against, as we will see with the following claims. And while this adjustment will result in more jabs sold for Pfizer, this is a logistical nightmare. Giving two injections in a relatively short time to small children is already annoying, but three injections may be one shot too many for many parents. Especially if those jabs do not fit into the standard vaccination schedule for the normal childhood vaccinations. In addition, there is also a worrying thought behind it: If 2 injections do not work, it does not mean that the injections may not work, but that more injections should be given. Doubts about its efficacy are apparently out of the question.
Verdict on Claim 1: True
Claim 2 ‚Äď 6 children in the vaccine group got severe Covid, 1 in the placebo group
So the 2:1 ratio is relevant here. 6 children in the vaccine group vs. 1 in the placebo group then means a 3x greater chance of getting "severe Covid". First, let's look at the facts. The Pfizer report indicates: "Seven cases in participants 2-4 years of age met the criteria for severe COVID-19: 6 in the BNT162b2 group, of which 2 cases occurred post unblinding, and 1 in the placebo group." (page 38). So, Dr. Craig's pronunciation is correct. I do have trouble with the way she delivers it. By suggesting that you can say that the vaccine causes Covid, you open the door to the groups that are trying to undermine its message. I suspect that's not what she means at all. What she means, in my view, to say is that the definition of severe Covid, as used for this study, is ridiculous and in particular this one (see Appendix B): "Clinical signs at rest indicative of severe systemic illness (RR and HR, by age,1 SpO2‚ȧ93% on room air at sea level, or PaO2/FiO2 <300 mm Hg)'. This means that an increased heart rate and difficulty breathing was enough for severe Covid. If you've ever seen a small child crying, uncontrollably and struggling to breathe between lashes, then you understand that a child who has such a crying fit, and, coincidentally, also has a positive PCR test, should be diagnosed as a child with severe Covid. That's exactly what happened and that's what worked against them in the trial. Dr. Craig's message is factual: severe Covid is not a thing in children at all. Therefore, they chose a definition for severe Covid that was absurd and in reality does not indicate severe Covid. And if you read the trial results, I can agree.
Verdict on Claim 2: True, with comments
Claim 3 ‚Äď One child was hospitalized with a fever and a seizure. It was vaccinated
Here's something interesting. On page 37 in the report, a case is discussed of a 14-month-old child who was brought to the ER 9 days after testing positive because he had had a seizure. People with small children will know that they tend to always seem sick outside office hours (and spontaneously better again, when you are sitting in the waiting room of the hospital or was that just my experience?). The child had a fever of 38.4 degrees, was sent home and was completely symptom-free after 8 days. So not really anything to worry about. However, this doesn't seem like the kid Dr. Craig was talking about. It is probably referring to the so-called 'Executive summary' which reads: 'Only one of these severe COVID-19 cases (in a BNT162b2 recipient 99 days post-Dose 2) resulted in hospitalisation'. I have not been able to find anything else about this child. So I cannot confirm or deny that the child had a fever and a seizure. It could be that 2 things have been confused here. What is certain is that the only child hospitalized due to Covid-19 was vaccinated.
Verdict on Claim 3: Probably true
Claim 4 ‚Äď Children had a 30% higher chance of getting Corona after the first shot
To do this, we need to look at the effectiveness data, which we find in tables 19 and 20. I've copied them below.
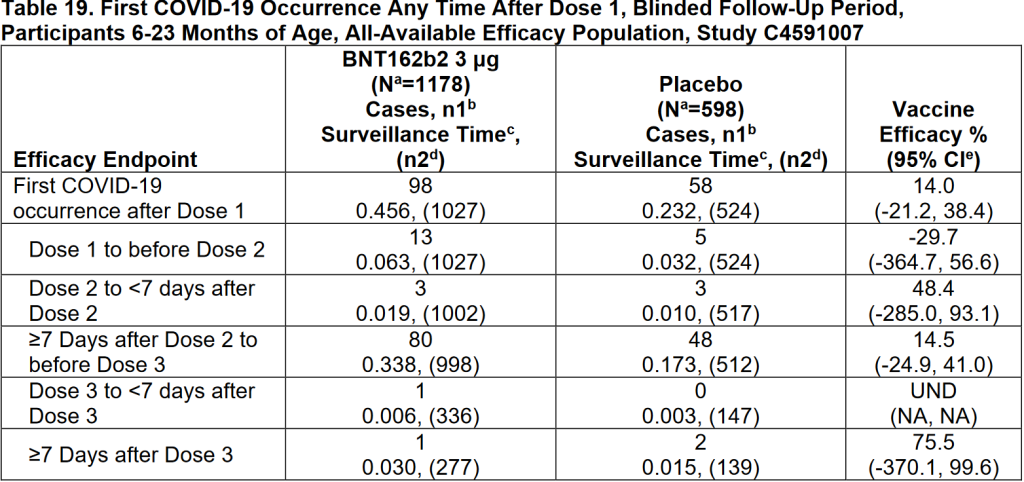
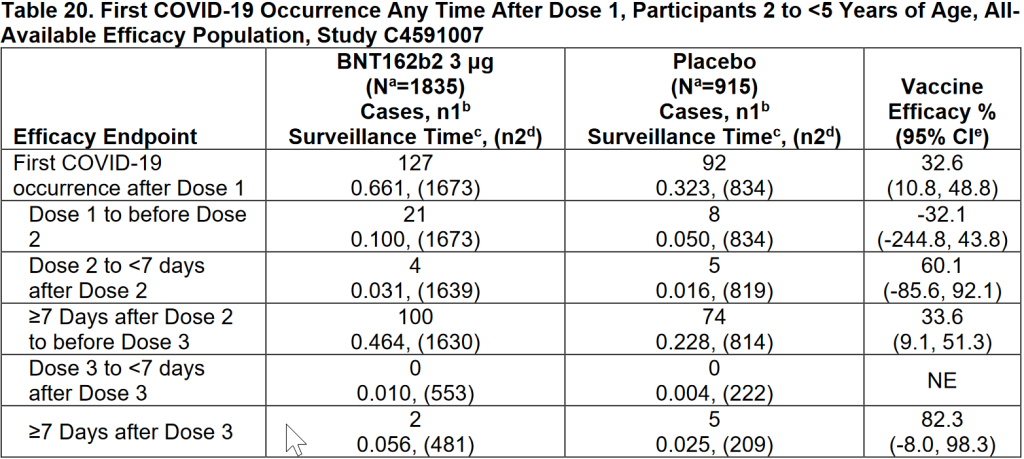
I had already suggested under claim 1 that the vaccine effectiveness, as shown by the results, for the first 2 injections was quite dramatic. For now, let's look at Dr. Craig's claim. Children between the ages of 6 and 23 months had a 29.7% greater chance of getting Corona in the first 3 weeks after vaccination 1 when they were vaccinated. For the 2 to 5-year-olds, it was 32.1%. Roughly 30%. According to the trial, vaccinated children did indeed have a greater chance of getting Corona within 3 weeks after vaccination 1. Of course, this is a bit of cherrypicking. In the entire period between injection 1 and injection 2, relatively more children in the placebo group had a positive test than in the vaccine group. If you take both groups together, you arrive at a vaccine effectiveness of 24.5%. That's very low, but it's bigger than zero.
Verdict on Claim 4: True, but with comments
Claim 5 ‚Äď Between the 2nd and 3rd doses, many children contracted Corona. These have been ignored
If you look at the tables, you can see that 187 children in the vaccine group tested positive and 122 in the placebo group. That's a lot of positive tests. Have these been ignored? That depends on your definition of ignored. They haven't been ignored when you consider that this result prompted Pfizer to change the protocol and add a third jab. However, in Pfizer's reporting with the vaccine effectiveness they mentioned, only the last percentages from the table are mentioned and in that sense they have indeed been ignored. So I agree with Dr. Craig on this.
Verdict on Claim 5: True
Claim 6 ‚Äď they have ignored 97% of all positive tests and based their vaccine effectiveness on only 3% of tests
As far as I'm concerned, this is a very important claim. If, as a manufacturer, you indeed ignore almost all positive tests, and then triumphantly claim that your vaccine is very effective, this smacks of deception. Is Pfizer guilty of this? The answer is: Yes. During the entire study, 450 vaccinated children tested positive compared to 300 children who received a placebo. So 750 in total. If you then base your vaccine effectiveness on just 10 positive tests, as Pfizer has done, you have ignored 98.7% of all positive tests. That's even more than the 97% that Dr. Craig mentions, but that 1.7% I give her. So what would have been the vaccine effectiveness if you had included all positive tests? That is ((300*2) ‚Äď 450 / (300 * 2)) * 100% = 25%. That's a different kettle of fish than the 80.3% claimed by Pfizer. The biggest problem with Pfizer's claim, however, is the number of tests it is based on. 3 in the group up to 24 months and 7 in the group from 2 to 5 years. From a statistical point of view, those numbers do not have enough significance to be able to make statements about and that is reflected in the uncertainty intervals. For the group up to 2 years old, it ranges from -370% to 99.6%. So it could be almost 100% effective, but your child could also have an almost 4x greater chance of getting Corona by vaccinating it. So that doesn't mean anything and it is, to put it mildly, unethical of Pfizer to make claims about vaccine effectiveness based on these numbers.
Verdict on Claim 6: True
Claim 7 ‚Äď In the 2 months that followed, 12 children contracted Corona again. 11 were vaccinated
This is an interesting claim. The story goes around that because vaccination trains your immune system for only one part of the virus (the spike protein), your immune system has difficulty building up good immunity against the entire virus. Even after infection. This would mean that not only natural immunity works better against reinfection than vaccine-induced immunity (this has now been extensively proven), but also that immunity after vaccination and infection is less good than immunity after infection alone. That's obviously bad news for everyone who has been vaccinated. If we look at what is stated in the trial results, we see the following (on page 38): In the group under 2 years of age, 6 children were reinfected. Three in the vaccine group and three in the placebo group. In the group of 2 to 5 years, 6 children were also reinfected. Five in the vaccine group and one in the placebo group. So a total of 8 in the vaccine group and 4 in the placebo group. Is Dr. Craig's claim false? That's right. Three of the children in the placebo group only became infected after the blinding had been lifted and they had still had 3 injections with the vaccine.
It is a striking observation. A vaccine critic might see evidence in this that the vaccine undermines your already built up immunity via infection. However, that would not be a fair conclusion. Just as the number of positive tests after the third shot was too low to be able to make any statement about vaccine effectiveness, this number is simply too small to draw conclusions from this.
Verdict on Claim 7: True
Claim 8 ‚Äď Control group vaccinated at 6 weeks: No medium-term safety check
We beginnen met de eerste claim: ‚ÄúKinderen werden voor 6 weken gevolgd voordat de blindering werd opgeheven en de placebogroep alsnog werd gevaccineerd‚ÄĚ. Hier lijkt iets serieus mis gegaan, want in het rapport staat: ‚ÄúParticipants enrolled prior to implementation of protocol amendment 6 (N=3,883; February 1, 2022), were able to be unblinded at their 6-month post-Dose 2 visit, and those originally randomized to placebo were offered BNT162b2 vaccination at the age-appropriate dose level. Participants
enrolled after implementation of protocol amendment 6 will be unblinded at their 6-month post-Dose 3 visit, and those originally randomized to placebo will be offered BNT162b2 vaccination.‚ÄĚ
The children were therefore followed for 6 months instead of 6 weeks before the blinding was lifted. Of course, that makes a difference. However, the fact is that the placebo group was vaccinated after 6 months. At least, the report does not mention children in the placebo group whose parents refused the vaccine after these 6 months. We have also seen this vaccination of the control group in the other trials for their Corona vaccine. Vaccinating your control group is very unusual. After all, it means that you can no longer collect long-term data. The argument with which this has been done in the trials for the Corona vaccines is that it would be unethical to deprive these test subjects of these vaccines in the middle of a pandemic. With this argumentation, you can ask serious questions in this trial. The vaccine effectiveness in the trial turned out to be very moderate and the only child who ended up in hospital with severe Covid was also vaccinated. In addition, there are questions about the safety of these vaccines, especially in the longer term. A skeptic will therefore say that this abolition of the control group and thus making it impossible to collect long-term data on safety in a real trial suits Pfizer very well.
Verdict on Claim 8: Partly true
Immunobridging
With this, we have established that Dr. Craig's claims about the trial are largely correct. However, that doesn't tell you the whole story. She did not mention immunobridging at all. Yet this was officially the only thing that had to be demonstrated and also the reason that Pfizer had to add a third jab: "Immunogenicity analyses of GMTs and seroresponse rates at 1 month after Dose 2 were evaluated for both age groups, and the pre-specified immunobridging success criteria (below) were not met for participants 2-4 years of age. Therefore, a third primary series dose was added for both age groups with protocol amendment 6."
Now, immunobridging will be an unfamiliar term to many. In this context, presentation of the WHO it is explained quite clearly. In short, if you can demonstrate that a candidate vaccine gives the same immune response as an already approved vaccine, the vaccine can be approved on that basis. If a vaccine gives the same immune response as a vaccine whose effectiveness has already been proven, you are done. This ensures that manufacturers do not have to set up an extensive (and therefore expensive) trial every time the vaccine is adjusted. It is also used to avoid having to extensively test drugs on children, because you simply prefer not to do large-scale trials on children. So there is nothing wrong with this in itself.
However, it becomes a problem when you are dealing with a rapidly mutating virus, so the trial results on which the original approval is based have only limited predictive value for effectiveness with the current virus variant. And that's the situation we're seeing right now. In today's world, 2 shots with the Pfizer vaccine offer little to no protection against infection with the Omicron variant. Protection against serious illness has also plummeted and, if we are to go by the hospital figures from Sciensano, the Belgian National Institute for Public Health and the Environment (RIVM), it is even quite negative. The chance of ending up in a Belgian hospital as a double-vaccinated person with a positive test is considerably higher than for someone who has not been vaccinated (see page 25 of this report). If the original trial had continued and people had been followed for longer, the provisional approval would have been withdrawn long ago in the case of a well-functioning medicines authority. The fact that the vaccine triggers the same immune response in children as 2 injections did in adults does not speak for the vaccine.
Administratively, however, you can still get approval for your vaccine with a sufficiently large immune response in children against the Wuhan variant. It makes sense for Pfizer to take advantage of this. They simply see an additional market. It is reprehensible of the drug authorities to allow this, because it should be clear that this measured immune response in children says nothing at all about the effectiveness of the vaccine.
Side effects
Dr. Craig doesn't talk about side effects that occurred during the trial in her video. I came across four of them while reading:
Three parents have withdrawn their children from the trial after they experienced side effects. One child developed a high fever (> 40 degrees). One child has a severe attack of eczema and one child has a long-term fever. A child who remained part of the trial had a very high fever (40.8 degrees) for 5 days. These side effects have been determined to be a result of the vaccination.
Conclusion
As Dr. Craig reported and proven in this fact check, there were significant irregularities in Pfizer's trial in children aged 6 months to 5 years. The trial was started with the idea that 2 jabs would give enough immune response to prove immunobridging. When that turned out not to be the case, a third shot was added, but not before the blinding had already been lifted in more than 2/3rds of the participants, the control group had been given the vaccine and thus removed from the trial. After 2 injections, a vaccine effectiveness of only 14.5% was achieved for children up to 2 years of age and 33.6% for slightly older children. In the limited group that was left after the 3rd injection, there were too few positive tests to achieve statistically significant results. This did not stop Pfizer, when the few positive tests that were available to them turned out to be favorable for them, from flaunting high vaccine effectiveness in their press statement. This is nothing but pure deception.
The FDA's approval of the Pfizer vaccine for children aged 6 months to 5 years, is based on immunobridging found. This means that the same immune response has been observed in children as in the adults in the original trial (with 2 injections). However, we now know that these two jabs do not offer any protection against Omicron. Recent hospital data from Belgium even points to negative protection against severe Covid when someone is double vaccinated. It therefore points to gross negligence on the part of the FDA to give immunobridging-based approval.
However, the real question is, why would you want to vaccinate children against a disease that poses no danger to them at all with a vaccine for which there is insufficient safety data? Small children have never been a factor of concern throughout the Corona pandemic. In both the Netherlands and Germany, not a single child in the age category of this trial has died from or with Covid. Emergency Use Authorization is intended for a situation in which very serious illness or death can occur if a drug is not authorized. That is not the case with these children at all. On the other hand, there are serious concerns about the safety of the vaccinations. The fact that this trial was approved by the medical ethics committee at all therefore indicates the failure of these committees. Dr. Craig indicates that those responsible have a lot to explain. I can only wholeheartedly agree with that.
Infant and Covid mortality in the U.S.
Misinformation about the risk to children will have helped parents to participate in the trials. For two years, the CDC has published heavily exaggerated figures on infant mortality, which have been widely reported by respected media outlets. This announcement has been published after two years corrected, we read in BMJ.
An impression of reality:
- only 0.05% of all covid deaths in America were between the ages of 0-5, while that age group makes up 8% of the population. So they are 160 times less likely to die from Covid than average.
- There are 23.4 million children aged 0-5 years in the United States. Of these, a total of almost 600 died from/with Covid over a period of 2.5 years, which is 0.002%.
- In Amerika overlijden er in deze leeftijdscategorie jaarlijks ca. 3.500 kinderen.
- Also the news that a large part of the children within 1 year na covid-infectie de diagnose ‚ÄúDiabetes Type 2‚ÄĚ kregen zal ouders ongerust hebben gemaakt.

0 reactions