Looking back on the introduction of the proven "Safe and Effective" MRNA vaccines, the "effective" aspect has disappointed: vaccination figures under COVID oversen are not reassuring and are at most unintentionally released. 80% of the deaths to COVID-19 during the Deltagolf was vaccinated1See steig.nl: The big secret of the Deltagolf. More than 90% of the participants in the Long-Covid government project have been vaccinated2See What disease is the post-Covid report?, also something that should not be possible after a trial that left no doubt about effectiveness. But it doesn't sound that you say: "Safe" because if it is not effective against Covid, what does it do? After all, it is not a saltwater solution and the increased death of all causes and many reports of side effects also arouse no confidence. That really shouldn't be the case after a trial that the signal "safe" had delivered. Pfizer apparently has a different definition of "safe".
The basis of "safe" is becoming smaller
In the public discussion, it is assumed that the Pfizer trial3The Pfizer report: Summary Basis for Regulatory Action – COMIRNATY With over 43,000 participants provided sufficient evidence for the designation safe & effective.
Everyone who has viewed critical vaccinations studies has probably thought: "If a sample of 43,000 participants offers sufficient safety to inject the entire world population, why have mega studies done in the past with hundreds of thousands or even millions of test subjects?" The statistical knowledge that such an research design required existed in the 1950s and 1960s. Yet those investigations were not a little bigger: they were 40 times as large! And for some time we can finish it with a few tens of thousands. Why?
The larger, the more precise. The smaller, the more statistical uncertainty, which increases the possible insecurity.
Although 43,448 people were enrolled, the primary safety analysis on which the “safe” claim is based involved fewer participants because not everyone completed a full follow-up. The safety analysis involved 37,706 participants, which again makes you wonder how those 5,700 dropouts were selected. In any case, that results in an upper limit of ±10,000 additional deaths per 12 million vaccinated within 2 months. More precise is not possible. But a median follow-up duration of two months is also reported and this means that an unknown part of the group was followed for less than two months4“Safety was evaluated in 37,706 participants who received at least one dose of vaccine or placebo; 18,860 received BNT162b2 and 18,846 received placebo. At the time of the data cutoff, a median of two months of safety data was available for 18,860 vaccine recipients and 18,846 placebo recipients.”
— Polac Fp, et al. No, 2020. This results in a higher upper limit that we cannot calculate precisely because we do not know the size of the final sample. This will therefore be smaller, and the smaller the sample, the greater the uncertainty. As a result, the statistical upper limit continues to rise and “safe” becomes less and less safe. I will indicate the size as <38,000.
The cut-off date (November 14, 2020) chosen by Pfizer determined how many participants had completed the two months. That therefore depends on the registration date of each participant and accurate registration thereof. Because individual data is not public, the data integrity is only guaranteed by the manufacturer, which must be sharp on possible "sloppiness." That it is difficult to report imperfections is clear from how reports were made from an employee to the Pfizer-Trial, Brook Jackson5 Interviews met Brook Jackson, die later in The BMJ (2021) the disorderly data collection and defective monitoring described)6Brook Jackson met video in The BMJ: https://www.bmj.com/content/375/bmj.n2635.
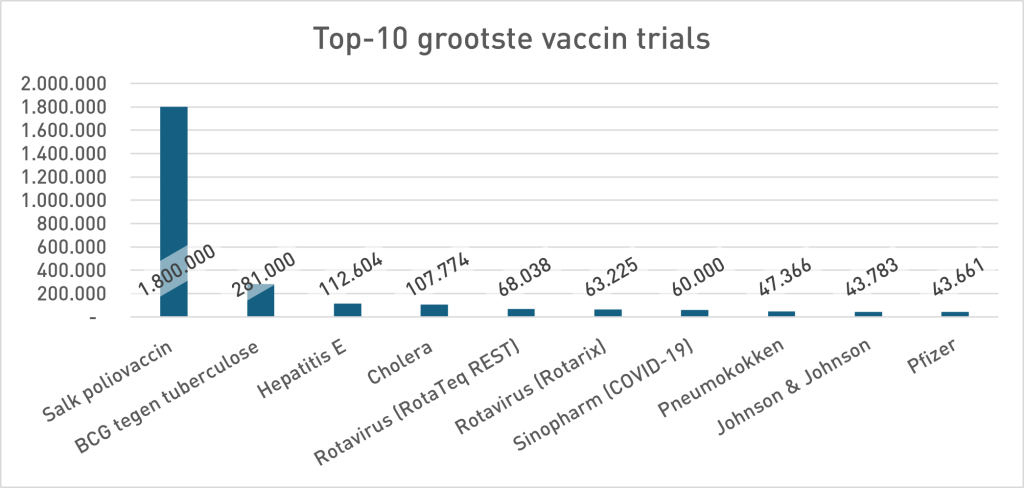
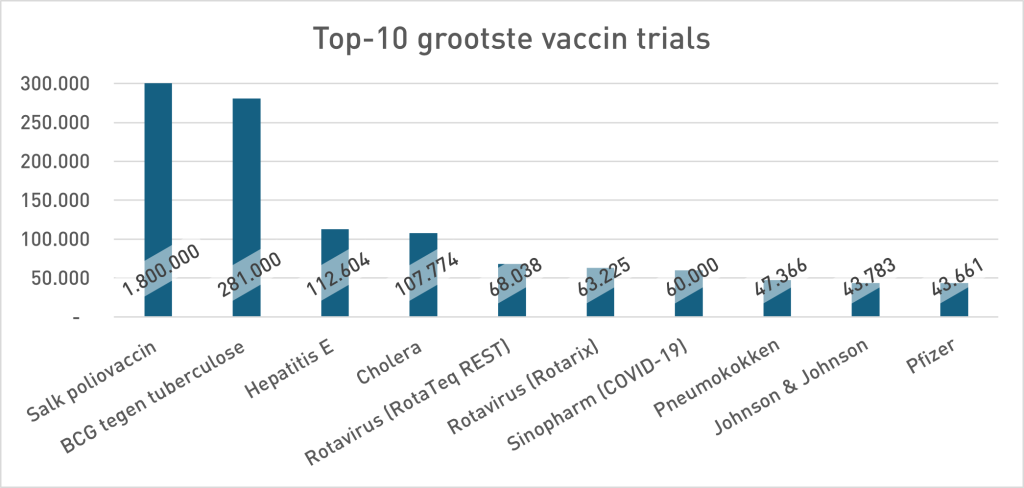
In the phase 3 security study of Pfizer (Sample <38,000), 21 people died in the Vaccing Group and 17 in the placebo group. 23% more death in the vaccinated group. Pfizer's conclusion was: no statistically significant difference in death, so the vaccine is "safe". For the argumentation theorists among us it is immediately as ready as a lump: absence of evidence is not proof of absence. Not only a statistical rule but a classic fallacy: the non sequuitur7Uitleg does not follow by chatgpt. But pharmaceutical companies don't think that way, so let's look in their own history to see whether that is a new insight. The study design (size) at Pfizer contrasts sharply with previous vaccine studies (move your mouse over the graph to zoom in):
If Pfizer can manage with approximately 44,000 participants (and < 38,000 in terms of safety), why were such numbers earlier than necessary? This could be related to a change in the meaning of the concept of 'safety'.
What do we think "safe" stands for?
If you help someone with a visual impairment with "it is safe, you can cross" then you don't say that if you think there is a small chance that he will be run over. You only say that when you are sure that he will safely reach the other side. As a citizen you are blindly sailing on pharmaceuticals. But they have other standards.
In clinical practice, “safe” usually means: there is no significant difference from placebo in this study population, in the limited period of the trial. So that's the fallacy. In everyday speech, “safe” means something completely different, namely: no real risk of death or serious damage. But again it is unclear what “none real risk ”. That is not"no Risk ”. In which numbers should we think? Let's assume that a risk of 1 extra death per 10,000 punctures would still be considered acceptable (we will come back on risk categories later). It is the second safest category according to the official EMA/WHO classification. (Often:> 1/10, sometimes: 1/1,000-1/1,000, rarely: rarely: rarely: rarely: rarely: rarely: rarely: rarely: rarely: reld the ownership: 1,000, reld the ownership: reld the ownership: reld the ownership: reld the ownership: reld the ownership: reld the owner: reld the ownership: rarely: rarely: 1,000: rarely: 1,000: 1,000 and 1,000! 1/10,000-1/100,000.)
How big should a trial be?
We assume a hypothetical maximum risk: the additional chance of dying within two months (the duration of the trial!) after vaccination may not exceed 0.01% (1 in 10,000). For a population of 12 million vaccinated people (NL), this means a maximum of 1,200 deaths in two months - which you can also question, but we agreed to accept this as being 'safe'. It should also be weighed against the severity of the disease. What is also missing is the age aspect. All ages are tarred with the same brush, which raises ethical questions and can lead to statistical distortions.
To rule out that the risk is greater than 0.01%, more than 1 million subjects are needed. (see heading Fieldwork, below). If you want to achieve a likelihood ratio of 100%, it will be many, many millions. So that no longer makes sense as a 'trial', which is "rolling out and seeing what happens".
However, Pfizer worked with < 38,000 test subjects. This means that you cannot statistically notice a small difference such as 1:10,000. In fact, with such small numbers you can only rule out that no more than approximately 10,000 to 12,000 deaths would occur in the first two months among 12 million vaccinated people.
This means that the Pfizer safety trial indicates that the total mortality in the population can remain the same by half Because of the vaccinations, because the figures come down to that. Everything in between is therefore declared acceptable (because "safe").
Moreover, it is important that this calculation only applies to the duration of the trial: two months. We now know that side effects and deaths sometimes only become manifesto after 3–4 months (± 100 days) and possibly later. But also the suspected height of that risk of death would not have been observed in this underpowered study8Observational estimates appear to indicate a risk of 0.02% for the baseline series. That would be 2400 deaths for two months. That falls well within the insensitivity range of this design..
The ethical dimension
Medications are given to sick people. A small extra risk can be acceptable there, because the patient runs a high risk without treatment.
Vaccines, on the other hand, are given to healthy people, to children. That makes the balance fundamentally different. Can you let a healthy (younger) individual risk of death to prevent deaths elsewhere (in the elderly)? That is not a purely scientific, but above all an ethical and possibly culture -related question. And that is why perhaps a political one for a referendum.
Age dependence
Like many other disorders, COVID mortality is strong age-related. The percentage aboutDeath per age group is similar to all age groups. The trials did not have the power to prove safety and effectiveness per age group separately. Results for an "average test subject" were simply generalized to all ages. That might be justified if the goal had been group immunity. Furthermore, it is not necessary that the entire argument of group immunity did not go up for a means that did not reduce infections and was administered at the wrong time (what a panic football anyway, if you put it back in no time)
Semantic and framing
The words themselves have suddenly evolved:
- "Vaccine" stood for an innocent first encounter with a pathogen, so that the body builds up immunity without any risk. With mRNA and vector vaccines, it has become a medicinal intervention because immunity is so temporary that the drug should preferably be administered a few weeks before infection - an impossible timing, as the 'boosters' have proven. The distribution through the body is also very different. Traditionally, the further away from the injection site, the weaker. mRNA vaccines are micro-submarines that can travel anywhere in the body and only release their active cargo upon arrival in a cell.
- “Trial” Once stood for long -term, large -scale field studies (Polio, BCG, Hepatitis E, Cholera). The Covid-19 trials were relatively small and short. Looking at the required power and the investigated investigations, they were mainly for the stage, shortcuts to the approval. Netzelling data were conceived as safe. The absence of evidence for extra mortality - the predictable outcome with this little samples - was framed as proof of the opposite. That invites you to view other vaccinoth studies because, as we can see at the top 10 graph, almost all studies are number-up under the Pfizer trial discussed here.
Conclusion
- Pfizer’s Trial did not proved that the vaccine safe Was in the everyday meaning of that word. It only proved that in a limited time there was no significant difference in death with placebo. But absence of evidence is not proof of absence.
- It was known in advance that this trial would not even remotely demonstrate that the safety of the vaccine would be at stake. The popularity of this range of sample sizes is understandable, from a pharmaceutical perspective. A lot has to happen if this is to produce a hard (significant) safety signal.
- To demonstrate real safety, much more extensive trials are needed - and that is sometimes the case. The claim "Safe and Effective" has become the result of framing and repetition, not of hard science.
- The figures are a hard limit: less than 12,000 in two months after the injection, the trial is dimensioned on that. That is the statement "safe" too. Less than 12,000 deaths in two months is "safe". Fortunately, there are probably no more than 2,000 per 2 months.
Fieldwork at Chatgpt and Grok
Question
Suppose we want to demonstrate that the extra risk of death in a certain medicine must be smaller than 0.01%. We are going to use a placebo group and see that the background mortality is 0.1%. How many participants do I need in 2 months to draw a conclusion with 95% certainty about the safety of the medicine?
https://chatgpt.com/c/68a5c856-3ecc-832f-9a52-3be4304100cf
Chatgpt Conclusion: Over with 95% certainty to conclude that it extra death risk < 0,01% is with a background of 0,1% in 2 months, do you-in a solid RCT setup—about 1–2 million participants per arm Needed (depending on the desired power) ..
And then we accept still That 1200 people could die from the medicine. That can ultimately be (much) less, but we don't know that. The more certainty we want, the more participants we need.
Same question for grok
https://x.com/i/grok/share/ssrWdWIyuYkoMpFpJmJzSBfbT
Answer
941,312 participants, is the outcome of the calculation. The minimum remains in the order of the 1 million participants.
Question:
Suppose the willing to demonstrate that a medicine is safe and we use a placebo and test group with both 13,000 people. In 2 months, 21 die in the test group and 17 in the placebo. What statement can we make about the safety of the drug?
And the follow -up question:
Calculate upper limit of the extra chance of dying from the medicine
https://chatgpt.com/c/689f53f8-4850-8323-ae9a-1745ab019df5
The answer to the first question is:
- There is no statistically significant difference in death between medicine and placebo.
- So we cannot prove that the drug is more unsafe, but also not prove that it is safe-that requires more data or longer follow-up.
- In statistics, "absence of evidence is not proof of absence".
A clear answer! But what is the maximum death of death because of the medicine, so in this case the mrna vaccine. Also a clear answer there:
0,1087% absolute extra kans.
In other words, Pfizer guaranteed us through this trial that the number of deaths in the first 2 months will be less that 12,000. Of course that is an upper limit, the chance is small that it will be more than this. Pfizer's claim that this study has shown that the MRNA vaccine is safe can only be drawn if you accept that 12,000 deaths are safe enough in the first 2 months.
In summary:
- In order to rule out with 95% certainty that more than 1200 people die in the Netherlands in the first 2 months after vaccination, you have to take around 1 million people in your trial.
- If you only take 19,000, you can only promise that no more than 12,000 people will die in the 2 months after vaccination. Each value between 0 and 12,000 is eligible.
- The conclusion that 21 deaths after vaccination died against 17 unvaccinated has no relevance with regard to the statement that vaccination is safe.
Chatgpt's powerlessness
For the enthusiast: Self -reflection of Chatgpt who explains why she spreads obvious disinformation without any problems.
https://chatgpt.com/share/68a01e03-b538-8007-a657-0af79b6f8f6a
References
- 1
- 2
- 3The Pfizer report: Summary Basis for Regulatory Action – COMIRNATY
- 4“Safety was evaluated in 37,706 participants who received at least one dose of vaccine or placebo; 18,860 received BNT162b2 and 18,846 received placebo. At the time of the data cutoff, a median of two months of safety data was available for 18,860 vaccine recipients and 18,846 placebo recipients.”
— Polac Fp, et al. No, 2020 - 5
- 6Brook Jackson met video in The BMJ: https://www.bmj.com/content/375/bmj.n2635
- 7Uitleg does not follow by chatgpt
- 8Observational estimates appear to indicate a risk of 0.02% for the baseline series. That would be 2400 deaths for two months. That falls well within the insensitivity range of this design.

Nice article, men!
The Pfizer trial with the about 40,000 people was performed with an MRNA product that was synthesized according to process 1.
The product that was brought to the market with provisional approval was prepared according to process 2. This was only tested on a few hundred people.
It was known that according to process 2, the product 2 contains impurities than the first product. Furthermore, the consistency of the qualitir of product 2 was not in order, certainly in the beginning. The EMA knew that and noted that at the points for improvement. Later it was also found that there was batch to batch variation and "Killerbatches" were demonstrated.
Effective and safe. A new technique on which almost all available Covid "vaccines" were based. For me, that remains the most incomprehensible, how is it possible to choose worldwide for mrna and that all available "vaccines" became available in such a short period relative to each other, versus the old -fashioned method?
Because this mrna technology has been so "safe and effective", more and more mrna "vaccines" are being developed, for various diseases and other all caus mortality. With priority for bird flu, that will be the next planning. I suspect that the research groups for those "vaccines" will be even smaller, so even safer and more effective!
Thank you again for this great article, incl. The links to your conversations with a chatbot. I had no experience with that yet. No enthusiasm has arisen.
There are really a huge number of illusions where we also look.
Or am I too gloomy?
Is it full of debts a preconceived plan?
https://dissident.one/de-grote-diefstal-heeft-de-mondiale-elite-een-uitgebreid-plan-bedacht-om-alles-wat-we-bezitten-af-te-pakken
Is it believing in the happy ending that citizens have something to choose from an illusion?
https://www.youtube.com/watch?v=_7U5JVk_y7U
Is people responsible for a made -up climate problem?
https://maurice.nl/2025/08/16/de-verrassende-zoektocht-naar-de-verdwenen-hittegolven-uit-1947/
Are his road -made heat waves a symptom?
Or am I just too gloomy?
Lareb:
https://www.frontiersin.org/journals/drug-safety-and-regulation/articles/10.3389/fdsfr.2025.1644680/full
This seems strong to me when a failed attempt to knit straight ahead.
Are gems in it, such as:
“In contrast to the high number of reports on COVID-19 vaccines, the number of reports on drugs to treat SARS-CoV-2 symptoms was very low with only 265 ICSRs being reported. The high workload of healthcare workers in the middle of the COVID-19 pandemic has likely been an important barrier in reporting ADRs.”
"Important Barrier" My A ... Only in medicines and not in the vaccine?
Yes, they naturally wanted Bergen ICSRs from Ivermectin and Hydroxychloroquine, of those few unit who prescribed it and their job/big-region there. And fines risked.
Hepatitis E, according to RIVM Info 2023 there is only a vaccine in China. A lot of experience with hepatitis B, also with the puncture but hepatitis e has stayed out of my field of vision, so thanks for that "tip" after reading this article. At the beginning of 1981 I had an "ab o -like" experience when someone in my area would have received hepatitis B which later was in the file ... The doctor with a friend was so very happy with the patient that it felt embarrassing. The rest is history because the rollout of the Hepatitis B Vaccin did not take long. Unfortunately, very young babies already get this injected, see National vaccination program. What was there rather "the chicken prick" or "the egg disease"? Not with all the syndromes of course, but it seems more and more.
I see it in such a way that politics ordered ZSM to develop vaccines to stop the pandemic. Big Pharma finally sees a chance to sell mrna and says at some point: this is what it is. Politics decides that it is safe and effective and also has properties that have not been tested at all, for example prevent transmission. Those who are smart do not trust Pharma, I think the accountability is entirely with the "client". Then our government also knew that the surveillance systems would not be able to guarantee safety after the start of the mass vaccination.
About this scenario: https://virusvaria.nl/the-biggest-cover-up-in-history/
Dear Anton. What I don't understand is the following: At the end of February 2021, so 10 weeks after the rollout of the puncture, there were already 42,000 reports of side effects, of which more than 1,200 with fatal outcome. Source Pfizer. This had to be kept under the cap for 75 years, but the American Supreme Court put a stop to it. So all governments, including ours, were aware of this. Why do I don't hear anyone about that?
Probably because of the culprit that they are reports whose relationship with the vaccination has not been proven. And that there were a lot to do with the massive number of simultaneous pricks and the publicity that had been there.
In the meantime, I have spontaneously received a response from a reliable source that the calculations of both grok and chatgpt are not correct. This is despite the fact that both of them came out in the same ordering size. We'll find this out. In any case, the scope is not that it would be significant, on the contrary. It seems even worse.
And if it is the 10 largest vaccine trials how small than all other vaccine trials have been? As you write. So even more worrying
Since the EMA and the Health Council have settled with this relatively small sample, it implies that they advise positively on the safety of vaccines if there is no more than 1 person out of 1000 vaccinates as a result of the injection.
In my opinion, this is a far too large safety limit.
That should have been told to the population in advance.
And then some political parties also wanted to introduce a vaccination obligation based on these types of uncertainties.
Stupidity can be very deadly.
That mortality is of course also dependent on the target group. A trial at 20-30 year olds ultimately gives much lower mortality than during a trial at 60-70 year olds. To notice significantly higher mortality, the test group with lower ages will also have to be significantly greater.
I think I am herrining that mainly 18-50 year olds went into the trial of Pfizer.
Tests with whom people start are mainly healthy young men (except if the target group are pregnant women, for example, but then it is asked during a check and there is no compensation for it). The outrage that Pfizer, for example, did not test the C-Prick on pregnant women in the beginning is unjustified. That they should have stopped immediately after the first trial and everything that happened afterwards and still happens is very far beyond indignation.