Do the safety and effectiveness data from clinical trials C4591001 support the approval of a COMIRNATY booster dose, to be used in individuals 16 years of age and older, at least 6 months after completion of the primary series? That was the first question raised in the FDA Hearing about the boosters. I give an impression of the video registration because I think that hardly anyone has watched it.
It has become a fairly long article based on a video that lasts more than 8 hours. First, the contributions that pass by during the Open Public Hearing.
The opinions in the Open Public Hearing Session
In the Open Public Hearing session, a number of experts and stakeholders (doctors and activists) each get three minutes to explain their point of view. Below is the youtube time code and most important keywords per head. It is tempting to only look at the medical status, but it is actually about the validity of the views.
Pfizer has researched the booster shots. Do the results provide the rationale to approve the booster shots for everyone aged 16 and older? (Keep in mind that the EMA approves boosters from the age of 18.)
Rajesh Gupta Ph.D. : Strongly advises against. The presence of antibodies is not a reliable target. Trials have not investigated infection. Little sense unless with the very vulnerable. Against vaccinating 16+.
Ben Newton – Approval requires 50% effectiveness, so boosters are desperately needed to stay above 50% given the decreasing effectiveness! Talks about "The right to be vaccinated" and "Every unvaccinated person has the same R0 value as an anti-vaxxer". [Is this a scientific approach...?]
Dr. Jessica Rose: Viral immunologist. Points to the sheer amount of side effects in VAERS: more than all vaccines combined in the past 10 years. Especially unacceptable for children. Variants only become more dangerous because of the booster shots. So don't do it.
Professional. Retsef Levi (URL)– Not satisfied with monitoring for side effects in Israel [research is being done, will be corrected later]. Herd immunity is not possible. Still high infection rates and mortality is increasing despite the vaccines. First determine research and strategy. Until then: don't do it!
Dr. Joseph Fraiman – Need for larger trials. Gain experience with Covid and with the vaccines. For people in their twenties, it is not possible to prove that covid is more harmful than vaccines. Too little evidence to be able to give the boosters to everyone! Also look at other medications.
Steve Kirsch – Trial showed that there were 4x as many heart attacks in vaccinated people. 20 deaths among vaccinated people; 14 in the placebo group. Vaccines cost more lives than they save. Myocarditis occurred in about 1:1000 boys. After vaccination at 1:317. After the booster, he expects 1:25. Points to fraud in the trials. Very strongly against.
Dr. David Wiseman – Politicized topic. No studies, no proper effectiveness and certainly not about the boosters. In Israel, excess mortality increases with Booster roll-out. Menstrual problems. Gene therapy should be investigated for carcinogenicity for 5-10 years. In short: too little data, too many side effects, no insight into long-term effects, especially relevant for young people. Look at existing drugs! Against.
Kermit Kubitz – Mentions advantages: "Boosters" are misnamed, normal vaccination protocols also sometimes require months between administration. Boosters protect against variants and getting sick despite being vaccinated. Boosters protect against infection, including unvaccinated people. Worldwide delivery will come from cheaper, more conventional vaccinations and Biologic E vaccines are coming [I have taken these points almost verbatim because I don't understand everything but what I do understand is not true]. But in any case: Before the boosters
Dr. Peter Doshi – If there is a Pandemic of Unvaccinated people, why a Booster? There is no research capable of side effects of third dose. Furthermore, all warning voices from doctors and scientists are dismissed as "spreading misinformation". Worrisome situation, what is the FDA doing about it? Under these circumstances: Clearly against. Too many strangers.
Dr. Michael Carome – No risk/benefit profile for the boosters. Unclear to what extent side effects increase. Protection against serious illness unclear. Quotes Lancet. More important to vaccinate poor countries. Speaks of 'highly effective'.
Kim Witczak – Goalposts continue to be moved. Trials are severely insufficient, involve several hundred people. Absurd as a basis for a global roll-out. mRNA vaccines are never designed to stop infection or eradicate the virus. False pretenses: vaccines would be better than natural immunity, people were 'bribed' to get vaccinated, demonizing unvaccinated people. Again accusation of obstruction of free speech and certainly of doctors and scientists. Side effects are huge. The biggest medical experiment ever. So against.
Lynda Dee – Scientific basis is lacking. Discusses approval protocols. So too little data.
Dr. Meg Seymour – Security research is far too small and also not representative. Only 12 patients aged 65 and over, while that is the most important group. Ages 16-17 are modeled based on the results of 18+. Three months is also too short to signal effects. Methodological objections, not scientifically justifiable. So very much against.
Kathleen Cameron – Very pro-vax, wants rapid vaccines for the elderly and communities of color. Asks for an informed decision because vaccines are important and the impact of Covid-19 is terrible so we want to do everything we can.
Beth Battaglino – Promo speech for the activities of her foundation. No opinion to be made.
Brian Hujdich – Concerned about decreasing effectiveness, especially in connection with his own foundation that deals with HIV patients. Would like extra protection for HIV patients and others with a malfunctioning immune system. Before, to inoculate everyone aged 16 years and older so that HIV patients are protected.
Dr. Paul Alexander – Strong criticism of the evaluation and monitoring. Scientific substantiation is insufficient, too short-term. "We currently do not have the safety data". Too many red flags in animal testing! So strongly against. Under no circumstances vaccinate children! Don't!
9/17/2021: FDA Advisory Board advises "conditional"
On September 17, the FDA met to vote on approving the Booster shots. To be seen on this over 8-hour Youtube video. The video is a sometimes somewhat embarrassing sequence of old men shouting at each other that they are on mute and other technical problems, but more on that later. Pfizer gives online product presentations and research results with many graphs, slides, titers and anybodies with which Americans indicate antibodies. Questions are being asked about this by FDA members. Due to lack of time, it is sometimes necessary to little room for difficult questions
Toen ik die video destijds bekeek dacht ik ‘dat wordt niks’. De tegenstemmers wezen unaniem op het gebrek aan data van veiligheid en effectiviteit – en al helemáál waar het om kinderen en jongeren ging. (Zie hierboven de steekwoorden uit die eerste adviesronde van de Open Public Hearing Session, met tijdcodes naar de YT-vid.) De teneur was uitermate negatief.
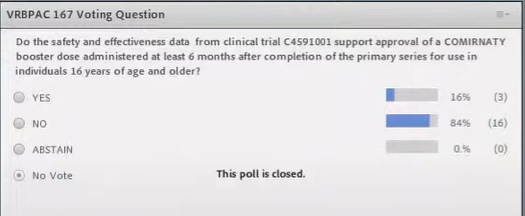
There were votes on the first request to get approval for the booster shots from the age of 16 three members for and 16 against.
Then to a second question. Plan B, so to speak: whether they could agree to booster shots for
- people aged 65 and over
- other high-risk groups, including people who have a high risk of infection due to their work

That turned out to be no problem at all, they voted UNANIMOUSLY IN FAVOR – which surprised me a bit after hearing the original objections.
22-9-2021: FDA conditionally allows
Five days later, on September 22, the FDA added the Booster shot to the Emergency Use Authorization for the Pfizer vaccine for the groups mentioned above.
Again in the Pfizer Fact Sheet the fixed condition that ALL serious side effects, regardless of whether the vaccine is suspected or not, must be reported.


Reporting must be done in the US to VAERS. There are research-based estimates that the underreporting in VAERS is a factor of 40. I doubt whether that factor also applies to the Covid vaccines because of the publicity surrounding the vaccines. Even if that factor is half, the conditions are not met in the US either. Apparently, this is not an obstacle to administering the product on a large scale.
4-10-2021: EMA is fine with it and promises to pay attention
After the FDA, the EMA can decide on application in Europe. The European Medicines Agency makes its recommendations for the boosters in Europe, a distinction is made between people with a weakened immune system and people with a normal immune system. She is thus leaving the age restrictions as set by the FDA.
EMA: In case of weakened immune system boosters only for vulnerable people
For the weakened, although there is no direct evidence that the ability to produce antibodies - as demonstrated with the booster - in these patients protects against COVID-19, it is expected that the extra dose could increase protection in at least some patients.
So nothing is weighed against possible side effects here, only possible effectiveness is looked at and the wish seems to be the father of expectation.
EMA promises to "continue to monitor the data that emerges on its effectiveness." What happens to the data on the side effects is not mentioned. The fact that these reports are also lagging behind in the Netherlands has now been old news.
EMA: with a normal immune system everyone from the age of 18...!?

De EMA concludeert dat voor de niet-kwetsbaren de boosterdoses mogen worden gebruikt vanaf 18 jaar en ouder. Ze vermelden wel nog dat het risico op inflammatoire hartaandoeningen of andere zeer zeldzame bijwerkingen na een booster niet bekend is en zorgvuldig wordt gecontroleerd (zie wat NL betreft de Lareb-link hierboven). Maar dan wordt in Nederland gezegd: “De EMA heeft het goedgekeurd, dus…”
EMA website: AUTHORIZED!

AUTHORISED! Not a word about mandatory reports or about conditions.
Elsewhere on the site, it is indeed reported that it is a conditional marketing authorisation "because the medicine meets an unmet medical need"; After all, it is necessary to fill the gap that is not filled by other medications. Therefore, it is not appropriate to little data on the market .
But then a nice green block with "AUTHORISED" really makes more of an impression!
Would a yellow triangle have been out of place? And not "AUTHORIZED" but "CONDITIONAL APPROVAL"?
But Pfizer already had Full Approval, didn't it...?
Anyone who reads the FDA's press release dated August 23 could understand that the Pfizer vaccine has long since been approved.
If you read a bit stricter, it says that the FDA has now approved "Comirnaty". formerly 'Pfizer-BioNTech CoOVID-19 Vaccine', the first Covid-19 vaccine.
The vaccine will also remain available (like Pfizer-BioNTech CoOVID-19 Vaccine) under Emergency Use Authorization, including 12-15 year olds and as a booster dose for immunocompromised people.
But I admit, it is very conveniently formulated and beneficial for the willingness to vaccinate.
How worrisome is it?
It doesn't make much sense to listen to the entire Q&A with Pfizer. The questioners would like to appear knowledgeable on Pfizer and ask long questions. They all invariably call each other "Doctor" which at some point takes on something colossal. Pfizer's answers are sometimes difficult to follow and yet people continue to watch with interest, presumably afraid that something is not right with their own connection. Only a technician dares to intervene a few times.
So much for the teaching, then also some entertainment. A small impression (I skip the screencam during the detailed question, at the bottom left of the screen I typed a comment here and there):
videolink: https://virusvaria.nl/wp-content/uploads/2021/11/FDA-pfizer-booster.mp4
The last hour is still informative, the members give an explanation of their vote. Het belangrijkste argument vóór is in elk geval om te zorgen voor minder besmettingen zodat de druk op de zorg afneemt. Men heeft kennelijk toch wat gemist qua bescherming tegen besmettingen.
Additional studies on this topic:
Waning Immunity after the BNT162b2 Vaccine in Israel https://www.nejm.org/doi/pdf/10.1056/NEJMoa2114228
Swedish study shows covid vaccines drop below zero efficacy on spread by about 200 days https://boriquagato.substack.com/p/swedish-study-shows-covid-vaccines?s=03
In it a link to:
Pre-print Effectiveness of Covid-19 vaccination against risk of symptomatic infection,
hospitalization, and death up to 9 months: a Swedish total-population cohort study
There was also a remarkably outspoken supporter who made himself heard a number of times: